By Peter Van Wyk and Leandro Castro
Bacterial diseases, especially those caused by Vibrio spp., are a leading cause of mortality in shrimp hatcheries. In the early days of shrimp aquaculture, antibiotics were the primary defense against bacterial outbreaks. Despite growing awareness of the risks associated with routine antibiotic use—particularly the emergence of resistant strains—many hatcheries continue to rely on antibiotics for bacterial control.
Beginning in the 1990s, however, the use of probiotics gradually gained acceptance, largely due to the pioneering work of Australian scientist David Moriarty (Fletcher, 2019). Today, probiotics are widely used in larval rearing protocols across shrimp hatcheries worldwide.
Water-based probiotic application
The application of probiotic bacteria to rearing water can improve both shrimp health and water quality. Studies have shown that probiotics reduce ammonia levels (Kewcharoen & Srisapoome, 2019) and help inhibit the growth of pathogens such as Vibrio spp. (Decamp et al., 2008), thus promoting a healthier environment for larval development.
However, water based applications have notable limitations. Effective probiotic dosing through water can be expensive. A typical application rate of 3–5ppm/day of a 109 CFU/g probiotic results in only 3,000–5,000 CFU/mL. While these treatment rates are affordable, some studies have suggested that pathogen control may require concentrations closer to 106 CFU/mL (Ghosh, 2025), significantly increasing treatment costs. Even increasing the probiotic additions to 50ppm/day, the resulting treatment rate would only be 5×104 CFU/mL.
Another limitation is that probiotics delivered through water do not effectively colonise the gut of the shrimp larvae. While such treatments may reduce the overall Vibrio load in the tank, they do not prevent larvae from ingesting high doses of Vibrio associated with contaminated Artemia or organic material (Verschuere et al., 2000). Once ingested, these pathogens can infect the shrimp through the lining of the gut.
Targeted delivery of probiotics to the gut
A more effective method of protecting the shrimp from Vibrio infection is to deliver the probiotic bacteria directly into the gastrointestinal tract by including the probiotics in the feed. This targeted approach allows probiotics to colonise the gut and establish a protective microbial barrier that intercepts pathogens at their point of entry.
Although direct measurement of colonisation in larval shrimp is difficult, a study by Zeigler and the University of the Philippines Visayas demonstrated that when juvenile Litopenaeus vannamei were fed with feed top-coated with Rescue probiotic (5×10⁶ CFU/g feed), Bacillus spp. were able to rapidly colonise the walls of the GI tract, reaching a density of 1×10⁷ CFU/g gut tissue in 7 days.
Once established in the gut, Bacillus probiotics limit Vibrio through several complementary mechanisms. They compete with pathogens for adhesion sites and nutrients, secrete antimicrobial compounds such as bacteriocins and lipopeptides, stimulate immune responses including phagocytic activity and upregulation of immune genes, and help maintain the integrity of the gut lining, reducing opportunities for pathogen invasion (Ghosh, 2025; Kewcharoen & Srisapoome, 2019; Zokaeifar et al., 2012;). Collectively, these effects make feed-based applications of Bacillus probiotics more effective than water treatments for long-term Vibrio control in shrimp hatcheries.
Probiotic strain selection
Bacillus species are well-suited for use in shrimp feeds due to their spore-forming ability, which facilitates packaging and storage. They are also highly adaptable, performing well across the broad salinity, temperature, and pH ranges common in hatchery environments. In addition, Bacillus spp. produce a wide range of antimicrobial compounds and enzymes that inhibit Vibrio, degrade biofilms, and interfere with quorum sensing (Shaheer et al., 2021; Ghosh, 2025).
However, not all Bacillus strains are equally effective. Some are particularly potent against Vibrio, while others are better suited for waste degradation or ammonia reduction. For this reason, strain selection is critical. Zeigler evaluated 21 Bacillus strains for their ability to inhibit four pathogenic strains of Vibrio spp. (Figure 1).
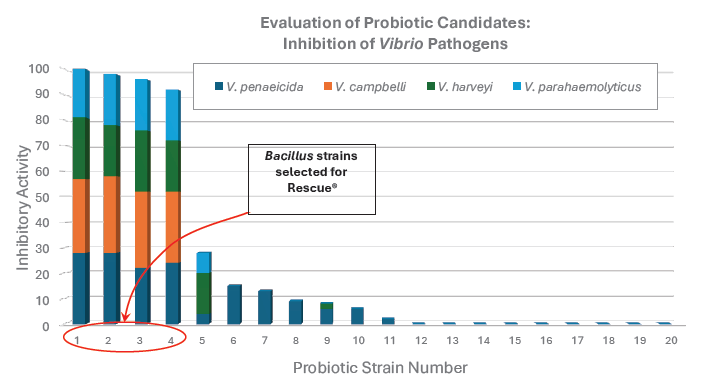
Figure 1. Selection of probiotic strains based on their ability to control four strains of pathogenic Vibrio.
The four best performing strains were included in the Rescue probiotic. Similarly, numerous Bacillus strains were evaluated for their ability to reduce ammonia levels and digest organic material (Figure 2), with five strains being selected for inclusion in the Remediate probiotic. This targeted strain selection ensures that each product delivers maximum performance in its intended use.
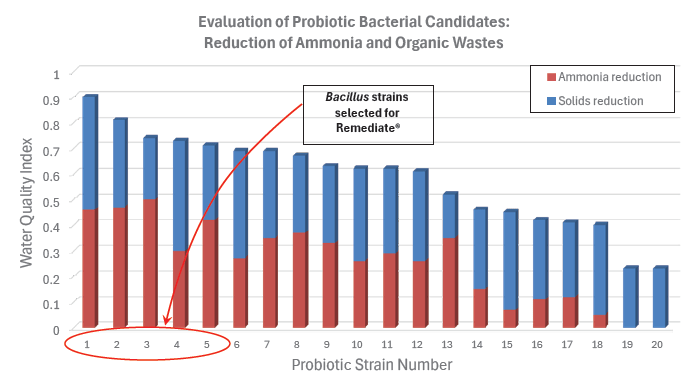
Figure 2. Selection of probiotic strains for ability to control ammonia and organic wastes.
Manufacturing challenges to probiotic inclusion
Despite the clear benefits of including probiotics in feeds, several factors limit widespread adoption. Maintaining the viability of the probiotics during the manufacturing process remains a major challenge. Most bacteria are unable to survive the high temperatures associated with processing. Although the endospores of Bacillus bacteria are relatively resistant to high temperatures, germinated spores are very sensitive to heat. The exposure of the spores to moisture in feed premixes is sufficient to activate the germination process. Activated spores are sensitive to high temperatures and do not survive. In nursery and grow out diets, probiotic bacteria can be mixed with oil and top-coated onto the feed pellets. However, this approach does not work well for larval diets, which, after pelleting, are ground finely and then sieved. Top-coating oil onto finely ground feed results in clumping of the feed.
An innovative solution for incorporating probiotics into hatchery diets
To address this problem, Zeigler developed a proprietary cold-process method for incorporating probiotics into liquid larval feeds. The resulting products, EZ Larva Ultra and EZ Artemia Ultra, preserve spore viability and allow for precise and consistent probiotic delivery.
Rescue, a blend of Bacillus strains selected for their anti-Vibrio activity, is enclosed within microcapsules for targeted gut delivery. Remediate, formulated to reduce ammonia and organic waste, is included in the liquid fraction of both feeds and disperses into the rearing water upon feeding. Both products deliver viable spores at efficacious levels when fed as directed.
In addition to probiotics, Zeigler’s liquid feeds also contain organic acids and Vpak, a blend of several functional ingredients that enhance immune function, improve nutrient utilisation, and increase resistance to oxidative stress. These functional ingredients help maximise the overall health benefits provided by the probiotic bacteria.
Proof of effectiveness
With rapid gut transit times in larval shrimp, in as little as 20–30 minutes (Jones et al., 1997), some skeptics questioned whether this allows sufficient time for the spores to germinate and colonise the gut. To test whether the probiotics in EZ Larva Ultra and EZ Artemia Ultra could colonise the larval gut and improve survival, Zeigler’s Aquaculture Research Center conducted a feeding trial comparing two diet formulations. The negative control diet contained no probiotics, while the other included Rescue in the microcapsules and Remediate in the liquid fraction. EZ Larva Ultra was fed as 50% of the diet during the zoea and mysis stages. EZ Artemia Ultra replaced Artemia nauplii in the protocol during mysis and post larvae stages. Rearing tank water was also treated twice daily with 5ppm of Rescue (5×104 CFU/mL/day) throughout the study. The larvae were challenged at PL9 by immersion with Vibrio parahaemolyticus AHPND at 5×104 CFU/mL for 24 hours. Survival was recorded after 72 hours (Figure 3).
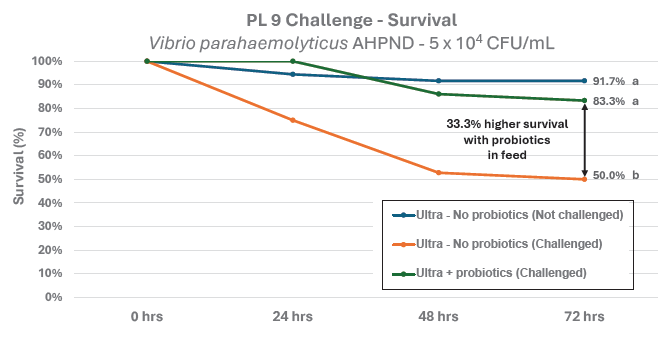
Figure 3. Results of a PL9 challenge with Vibrio parahaemolyticus AHPND. Survival was 33.3% higher in the treatment fed with feed containing probiotics, compared to survival in the treatment fed feed without probiotics.
The results were conclusive. Survival in the control group was 50%. In contrast, survival in the probiotic treatment group reached 83.3%, a 33.3% improvement. These findings clearly indicate that the probiotics delivered in the liquid feeds were capable of colonising the shrimp gut and significantly reducing mortality. Moreover, the improved protection suggests that feed-based delivery of the probiotics was more effective than water treatment alone.
Conclusions and economic implications
This trial, along with a growing body of research on probiotic mechanisms of action, points to a clear conclusion: delivering probiotics through larval diets offers a more efficient and biologically effective strategy for controlling aquatic Vibrio infections than applying them through water alone.
By targeting the gut directly, encapsulated Bacillus spp. colonise the gastrointestinal tract, inhibit pathogens, and stimulate immune defenses when shrimp are most vulnerable. Zeigler’s cold-processed liquid feeds maintain probiotic viability and allow for reliable, consistent dosing— advantages that are difficult to achieve with traditional dry feeds or water-based applications.
The implications for hatchery operations are significant. Even small increases in post larval survival can translate into substantial gains in seed output and revenue. Incorporating probiotic enriched diets can reduce mortality, improve larval quality, and decrease reliance on antibiotics, while enhancing the overall biosecurity of the production system.
References
Cao, Z., Yang, Q., Kang, A., Wang, G., Li, P., Qiu, G., Wang, J., Liu, C., & Sun, Y. 2025. Probiotic properties of Bacillus licheniformis HN318. Frontiers in Marine Science, 12, 1548955. https://doi. org/10.3389/fmars.2025.1548955
Decamp, O., Moriarty, D.J.W. & Lavens, P. 2008. Probiotics for shrimp larviculture: review of field data from Asia and Latin America. Aquaculture Research, 39: 334-338. https://doi.org/10.1111/ j.1365-2109.2007.01664.x
Fletcher, R. 2019. The godfather of shrimp probiotics. The Fish Site. May 14, 2019. https://thefishsite.com/articles/the-godfather-of-shrimp-probiotics
Ghosh, T. 2025. Recent advances in the probiotic application of Bacillus as a potential candidate in the sustainable development of aquaculture. Aquaculture, 594, 741432. https://doi.org/10.1016/j. aquaculture.2024.741432
Jones, D.A., Kumlu, M., Le Vay, L., & Fletcher, D.J. 1997. The digestive physiology and the selection of natural diets for crustacean larvae. Aquaculture, 155(1–4), 285–295. https://doi. org/10.1016/S0044-8486(97)00128-0
Kewcharoen, W. & Srisapoome, P. 2019. Immune-related gene expression and growth performance of Pacific white shrimp (Litopenaeus vannamei) fed a synbiotic diet and challenged with Vibrio parahaemolyticus. Fish & Shellfish Immunology, 86, 372– 383. https://doi.org/10.1016/j.fsi.2018.11.053
Shaheer, P., Sreejith, V.N., Joseph, T.C., Murugadas, V., & Lalitha, K.V. 2021. Quorum quenching Bacillus spp.: An alternative biocontrol agent for Vibrio harveyi infection in aquaculture. Diseases of Aquatic Organisms, 146, 117–128. https://doi.org/10.3354/dao03647
Verschuere, L., Rombaut, G., Sorgeloos, P., & Verstraete, W. 2000. Probiotic bacteria as biological control agents in aquaculture. Microbiology and Molecular Biology Reviews, 64(4), 655–671. https://doi.org/10.1128/MMBR.64.4.655-671.2000.
Zokaeifar, H., Balcázar, J.L, Saad, C.R., Kamarudin, M.S., Sijam, K., Arshad, A., & Nejat, N. 2012. Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 33(4):683-9. doi: 10.1016/j.fsi.2012.05.027.
Peter Van Wyk is Global Technical Sales Manager for Zeigler Bros. Inc. based in Florida.
Email: peter.vanwyk@zeiglerfeed.com
Leandro Castro is Senior Research Manager for Zeigler Bros. Inc. based in Pennsylvania.
May/June 2025 AQUA Culture Asia Pacific

